Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (30): 4515-4523.doi: 10.3969/j.issn.2095-4344.2016.30.016
Previous Articles Next Articles
Surface construction and biocompatibility of polymer materials as cardiovascular devices: modified tissue-engineered endothelial cells on the surface of polymeric biomaterials
Chen Bao-lin1, Wang Dong-an2, 3
- 1Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China
2Institute of Polymer Science, Zhejiang University, Hangzhou 310027, Zhejiang Province, China3Department of Pharmaceutical Sciences, University of Tennessee Health Science Center, Memphis, Tennessee 38163, USA
-
Received:2016-05-07Online:2016-07-15Published:2016-07-15 -
Contact:Chen Bao-lin, Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China -
About author:Chen Bao-lin, Professor, Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China
CLC Number:
Cite this article
Chen Bao-lin, Wang Dong-an. Surface construction and biocompatibility of polymer materials as cardiovascular devices: modified tissue-engineered endothelial cells on the surface of polymeric biomaterials[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(30): 4515-4523.
share this article
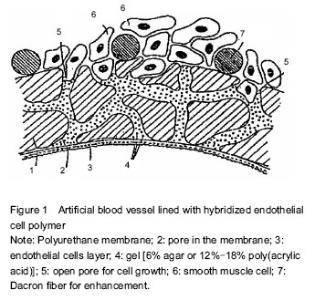
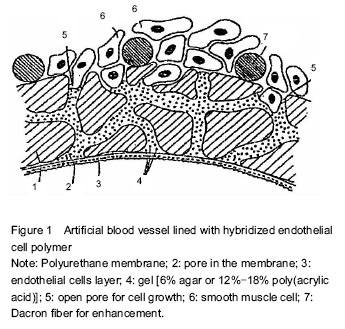
Functions of vascular endothelial cells Cardiovascular disease is initially induced by changes and damage of vascular endothelial cell function. During the detachment of endothelial cells, plasma component (lipid) infiltration, macrophage infiltration and intimal smooth muscle cell proliferation, damage and dysfunction of vascular endothelial cells occur earliest, and play a key role in the occurrence, formation and repair of cardiovascular disease. Vascular endothelial cells are a cell population as a layer covering the systemic vascular intima. Since this cell population has been successfully cultured in vitro, we have a very in-depth understanding of them. It is now thought that the endothelial layer is not only a barrier to blood and tissue, but also has a variety of other functions, such as reducing vascular permeability, regulating material exchange between tissues and blood, preventing disorderly invasion of plasma components and blood cells, resisting thrombosis, balancing anti-blood coagulation- fibrinolysis system and anti-platelet function, maintaining blood fluidity, adjusting vascular smooth muscle function, synthesizing and secreting factors related to vascular smooth muscle relaxation and contraction, and suppressing migration and proliferation of vascular wall cells[12-27]. Vascular permeability In vivo studies have observed that plasma component infiltration to the endothelium appears during endothelial cell division. To understand how lipid traverses endothelial cells, endothelial cells are co-cultured with low density lipoprotein. Results show that ApoB and ApoE receptors of endothelial cells are not associated with transendothelial transport of low density lipoprotein. In contrast, under histamine and thrombin stimulation, the cell permeability is evidently improved, and abundant low density lipoprotein is detected. It is thus clear that some biological factors (e.g., histamine), shear stress and newborn endothelial cells are associated with endothelial cell permeability. Antithrombotic effects In the 19th century, pathologist Virchow proposed three main factors of thrombosis including vascular wall damage, blood stagnation and blood anticoagulant system abnormalities. Under normal circumstances, endothelial cells exert antithrombotic effect and maintain blood fluidity. Nevertheless, with blood vessel wall impairment and the presence of cytokines, tissue factor is activated, antithrombotic ability of endothelial cells weakened, and thrombus easily formed within the blood vessel wall. The mechanisms underlying anti-thrombosis of vascular endothelial cells are as follows: (1) synthesis and secretion of anticoagulant factors, such as thrombomodulin, heparin-like substances and tissue factor pathway inhibitor; (2) synthesis and secretion of pro-fibrinolytic factors, such as tissue plasminogen activator; (3) synthesis and secretion of factors that inhibit angiogenesis and platelet aggregation, such as prostacycline and endothelial-derived relaxing factor. These factors keep the blood circulation and prevent thrombosis. Proliferation and migration of vascular endothelial cells Vascular endothelial cells can be induced to exert inhibitory effects on the proliferation of vascular smooth muscle. This mechanism can be affected by various factors secreted from above endothelial cells. Vascular endothelial cells also can proliferate and migrate under the stimulation of platelet, leukocyte-secreted migration factor and flow shear stress. This function is probably associated with arterial repair and neovascularization. Tissue-engineered endothelial cells on the surface of grafts Implantation of exogenous endothelial cells Herring (1978) proposed that endothelial cells implanted on the surface of inhibitors could increase the survival of grafts. Theoretically, the presence of a layer of confluent endothelial cells can elevate the antithrombotic ability of grafts, and prevent the occurrence of pseudointimal hyperplasia. The surface of the intact endothelial cells is negatively charged, which can reject platelet adhesion and exhibit obvious anticoagulant activity. Further studies have discovered that the patency rate of the grafts implanted with endothelial cells and antiplatelet is higher than that of grafts without endothelial cells. Moreover, the grafts implanted with endothelial cells show a strong antibacterial ability. However, these grafts show insignificant differences in lessening pseudointimal hyperplasia in the anastomotic area. In fact, high levels of platelet-derived growth factor (PDGF) can be detected in grafts implanted with endothelial cells, which is associated with the strong effect of PDGF on stimulating the migration and proliferation of smooth muscle cells. Thus, pseudointimal hyperplasia appears. One of the difficulties in endothelial cell culture is the relatively low density of cells used for transplantation, because of which cells cannot fully attach to grafts. In the process of trying to solve these problems, the researchers attempt to cultivate endothelial cells in two stages. The obtained endothelial cells are amplified in vitro until 100% confluence, and then implanted into the human body. Magometschnigg reported that vascular endothelial cells were implanted on fibrin-coated ePTFE grafts. The early patency rate increased by 20%, and the amputation rate decreased by 20% when above grafts were used in femoral-tibial bypass bridging. The disadvantage of this technique is the possibility of potential infections. Cell culture technology is more difficult and requires a second surgery. Another method to achieve the best cell density is to use microvascular endothelial cells. After small retinal vascular cells obtained by enzyme digestion are implanted on small-diameter Dacron grafts, a confluent endothelial cell layer will form with no thrombosis. As previously reported, a dog model exhibited an elevated 1-year patency rate. The current focus is to implant tissue-engineered endothelial cells on the synthesized grafts. Dichek has successfully transferred plasminogen activator genes into endothelial cells. Thus, modified cells could overexpress this fibrinolytic agent and enhance antithrombotic ability of grafts. In order to stimulate the proliferation and migration of endothelial cells and prevent excessive proliferation of smooth muscle cells, it is possible to make excessive secretion of growth factors and growth inhibitory factors from endothelial cells by transgene[28-34]. Stimulating re-externalization and angiogenesis of endogenous endothelial cells A mitogen and chemoattractant for endothelial cells can theoretically stimulate trans-interstitial capillary ingrowth, and enhance endothelialization of synthetic vascular grafts, mainly using fibroblast growth factor (FGF)-1(acidic FGF, aFGF) and FGF-2 (basic FGF, bFGF). Blood vessels can be formed in situ through the re-externalization of endogenous endothelial cells. This process mainly contains migration, proliferation and differentiation of vascular cells. Small blood vessels have been prepared successfully in the body. One of the specific models is: capillary endothelial cells induced by basic fibroblast growth factor project into collagen or fibrin matrix and form blood vessels. Angiogenesis induced by fibroblast growth factor is coupled with proteolytic enzymes produced by endothelial cells. The production of protease is a key step in angiogenesis, and provides a tool for endothelial cells to change extracellular matrix and to enter perivascular spaces. The structure of another vascular model in vivo is: cultured endothelial cells, smooth muscle cells and adventitial fibroblasts are paved in remodeled collagen and Dacron culture systems. Two weeks later, a confluent layer is formed to serve as an endothelial barrier. Different from the human body’s blood vessels, the tissue-engineered blood vessels requires a prosthesis to support and to maintain its structure, and do not contain elastin[11, 35-36]. Fixation of cell growth-promoting factor on the surface of polymeric biomaterials To realize the endothelial cellularization on the surface of polymeric biomaterials, bioactive factors should be introduced into the surface of materials. The bioactive factors mainly are proteins. The fixation methods of proteins on the surface of polymers are physical adsorption and chemical fixation. In addition, there are the newly developed ion implantation and self-assembled monolayer methods. Of them, the physical adsorption is relatively convenient, and contains two types: (1) electrostatic adsorption, for example, the heparin containing a negative charge is fixed in a positively charged site of the materials; (2) adsorption by intermolecular force, for example, adsorption between proteins and polymer molecules. In contrast, chemical bonding is more stable and overcomes the shortcoming in physical adsorption, while bioactive molecules cannot exert long-term effects on the surface of the material, and are easy to break away. The chemical fixation usually requires that the substrate surface has hydroxyl group, carboxyl group and amino-group. Therefore, the production of these groups on the surface of the materials by modification is the premise of fixation. Simultaneously, the fixation can cause the degeneration of proteins, and an optimal interaction between cell receptors and proteins cannot be established, so a spacer arm should be inducted in advance on the surface of a polymer. The chemical fixation of proteins usually contains two steps: polymer surface activation and reaction between activated surface and protein[37-53]. Protein fixation on the surface containing hydroxyl polymer Sulfonyl chloride method:Hydroxyl group activated by sulfonyl chloride generates reactive sulfonate, and further produces C-N or C-S bond by reaction with protein amine groups or thiol groups so as to fix proteins. Carbodiimide method: Hydroxyl group activated by imide generates imide-N-methyl ester, which contains leaving group. Thus, urethane bond is produced by reacting with protein aminogen to fix the protein. Diisocyanate method: Diisocyanate exerts a coupling effect. Hydroxyl group without activation reacts with one end of the coupling agent. One end of the coupling agent reacts to protein and fixes the protein. Protein fixation on the surface containing arboxyl polymer The activation of carboxyl group can be carried out by N-hydroxysuccinimide and 1-ethyl-3- (dimethylaminopropyl) carbodiimide. Protein fixation on the surface containing amino polymers Amino-group can be coupled to proteins through coupling agents diisocyanate, glycol aldehyde and epoxy resin. Irradiation fixation Irradiation fixation refers to fixation of proteins on the surface of polymers by a bifunctional coupling agent under the condition of irradiation. Light activator includes photosensitive azo, benzophenone and acrylate. Endothelialization of the material surface Endothelialization of the material surface is a new trend of anticoagulant study. The endothelialized surface mainly refers to pseudo-intimal surface or hybridized surface of endothelial cells and high polymer[54-56]. Pseudo-intimal surface refers to a layer of stable red thrombosis film forming on their interfaces when the material contacts with blood. The components of the film include plasma protein, platelet, fibrin and leukocytes. Thus, fibroblasts and endothelial cells grow on the film to form an intima similar to blood vessel wall, i.e., the pseudo-intima. At present, artificial blood vessels made of polytetrafluoroethylene with pseudo-intimal surface have been applied in the clinic[57-58]. It is worth noting that if the pseudo-intima of artificial blood vessels is very thick, nutrition will not support the body, cells will be necrotic and fall off, and coagulation will occur at the exposed part. A large number of studies have been carried out to control the thickness of the pseudo-intima. For example, porous polytetrafluoroethylene invades water-soluble polyvinyl alcohol to form a porous hydrophilic membrane on its surface and to reduce the adsorption of plasma proteins. The pseudo-intima formed on the artificial blood vessel wall is not a true vascular intima. The component and thickness of the formed protein layer cannot be controlled well. Although some improvements have been taken, the pseudo-intima on the artificial blood vessel wall does not achieve an effective compatibility to the transplantation site. Figure 1 shows the artificial blood vessel lined with hybridized endothelial cell polymer[59]. Endothelialization of material surface is a new trend of anticoagulant research. The blood vessel wall made of human endothelial cells is the only known blood-compatible material. Thus, we make hybridization of endothelial cells and polymers by imitating human blood vessels. That is to culture endothelial cells on the surface of material, finally forming a layer of endothelium. This would be a very ideal way for the improvement in blood compatibility of the material, especially the improvement in blood compatibility of small-diameter artificial blood "
| [1] Chen BL, Wang DA. Phase III study on surface construction and biocompatibility of polymer materials as cardiovascular devices: coagulant and anti-coagulant surface modification. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19(8):1277-1283. [2] Chen BL, Wang DA. Surface construction and biocompatibility of polymeric used for cardiovascular medical device. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18(21):3412-3419. [3] Chen BL, Wang DA. Surface construction and biocompatibility of polymeric used for cardiovascular medical device. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(34):6183-6292. [4] Chen BL, Wang DA. Hemocompatibility of biomedical polymeric materials-design of anticoagulatent materials. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(34): 6393-6396. [5] Yang ZM. Basic and Clinical Rusearch on Engineering. Chengdu: Sichuan Science and Technology Press. 2000: 239-247. [6] Ito RK, Rosenblatt MS, Contreras MA, et al. Monitoring platelet interactions with prosthetic graft implants in a canine model. ASAIO Trans. 1990;36(3): M175-M178. [7] McCollum CN, Kester RC, Rajah SM, et al. Arterial graft maturation: the duration of thrombotic activity in Dacron aortobifemoral grafts measured by platelet and fibrinogen kinetics. Br J Surg. 1981;68(1):61-64. [8] Gamble JR, Harlan JM, Klebanoff SJ, et al. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985;82(24):8667-8671. [9] Cameron BL, Tsuchida H, Connall TP, et al. High porosity PTFE improves endothelialization of arterial grafts without increasing early thrombogenicity. J Cardiovasc Surg (Torino). 1993;34(4):281-285. [10] Clowes AW, Kohler T. Graft endothelialization: the role of angiogenic mechanisms. J Vasc Surg. 1991;13(5):734-746. [11] Greisler HP, Ellinger J, Schwarcz TH, et al. Arterial regeneration over polydioxanone prostheses in the rabbit. Arch Surg. 1987;122(6):715-721. [12] Abbott WM, Cambria RP. Control of physical characteristics (elasticity and compliance) of vascular grafts. In: Stanley JC et al. (eds) Biologic and synthetic vascular prostheses, New York: Grüne and Stratton. 1982:189-220. [13] Hasson JE, Megerman J, Abbott WM, et al. Increased compliance near vascular anastomoses. J Vasc Surg. 1985;2(3):419-423. [14] Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104-114. [15] Eberhart RC, Munro MS, Williams GB, et al. Albumin adsorption and retention on c18-alkyl-derivatized polyurethane vascular grafts. Artificial Organs. 1987; 11(5):375-382. [16] Tsai CC, Huo HH, Kulkarni P, et al. Biocompatible coatings with high albumin affinity. ASAIO Trans. 1990; 36(3):M307-M310. [17] Rumisek JD, Wade CE, Brooks DE, et al. Heat-denatured albumin-coated Dacron vascular grafts: Physical characteristics and in vivo performance. J Vasc Surg. 1986;4(2):136-143. [18] Kottke-Marchant K1, Anderson JM, Umemura Y, et al. Effect of albumin coating on the in vitro blood compatibility of Dacron® arterial prostheses. Biomaterials. 1989;10(3):147-155. [19] Goëau-Brissonnière L, Mercier F, Nicolas M, et al. Treatment of vascular graft infection by in situ replacement with a rifampin-bonded gelatin-sealed dacron graft. J Vasc Surg. 1994;19(4):739-741. [20] Graham LM, Burkel WE, Ford JW, et al. Expanded polytetrafluoroethylene vascular prostheses seeded with enzymatically derived and cultured canine endothelial cells. Surgery. 1982;91(5):550-559. [21] Jarrell BE, Williams SK. Microvessel derived endothelial cell isolation, adherence, and monolayer formation for vascular grafts. J Vasc Surg. 1991; 13(5):733-734. [22] Kempczinski RF, Rosenman JE, Pearce WH, et al. Endothelial cell seeding of a new PTFE vascular prosthesis, J Vasc Surg. 1985;2(3):424-429. [23] Wakefield TW, Lindblad B, Graham LM, et al. Nuclide imaging of vascular graft-platelet interactions: comparison of indium excess and technetium subtraction techniques. J Surg Res. 1986;40(4):388-394. [24] Graham LM, Fox PL. Growth factor production following prosthetic graft implantation, J Vasc Surg. 1991;13(5): 742-744. [25] Jensen N, Lindblad B, Bergovist D. Endothelial cell seeded dacron aortobifurcated grafts: platelet deposition and long-term follow-up. J Cardiovasc Surg (Torino). 1994;35(5):425-429. [26] Zilla P, Deutsch M, Meinhart J, et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg. 1994; 19(3):540-548. [27] Magometschnigg H, Kadletz M, Vodrazka M, et al. Prospective clinical study with in vitro endothelial cell lining of expanded polytetrafluoroethylene grafts in crural repeat reconstruction. J Vasc Surg. 1992;15(3): 527-535. [28] Pasic M, Müller-Glauser W, Von Segesser L, et al. Superior late patency of small-diameter Dacron grafts seeded with omental microvascular cells: an experimental study. Ann Thor Surg. 1994;58(3): 677-684. [29] Greisler HP, Klosak J, Dennis JW, et al. Endothelial cell growth factor attachment to biomaterials. ASAIO Trans. 1986;32(1):346-349. [30] Gray JL, Kang SS, Zenni GC, et al. FGF-1 Affixation Stimulates ePTFE Endothelialization without Intimal Hyperplasia. J Surg Res. 1994;57(2):596-612. [31] Reilly CF, Kindy MS, Brown KE, et al. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989;264(12): 6990-6995. [32] Au YP, Kenagy RD, Clowes MM, et al. Mechanisms of inhibition by heparin of vascular smooth muscle cell proliferation and migration. Hamostasis. 1993;23(1): 177-182. [33] Kang SS, Gosselin C, Ren DW, et al. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery. 1995;118(2):280-287. [34] Gosselin C, Ren DW, Ellinger J, et al. In vivo platelet deposition on polytetrafluoroethylene coated with fibrin glue containing fibroblast growth factor 1 and heparin in a canine model. Am J Surg. 1995;170(2):126-130. [35] Greisler HP, Schwarcz TH, Ellinger J, et al. Dacron inhibition of arterial regenerative activities. J Vasc Surg. 1986;3(5):747-756. [36] Greisle HP, Dennis JW, Endean ED, et al. Derivation of neointima in vascular grafts. Circulation. 1988;78(3 Pt 2):6-12. [37] Cifonelli JA. Relation of chemical of heparin to its anticoagulant activity. Adv Exp Med Biol. 1975;52:95-103. [38] Castillo EJ, Koenig JL, Anderson JM, et al. Protein adsorption on hydrogels. II. Reversible and irreversible interactions between lysozyme and soft contact lens surfaces. Biomaterials. 1985;6(5):338-345. [39] Drumheller PD, Hubbell JA. Surface immobilization of adhesion ligands for investigations of cell/substrate interactions. CRC and IEEE Press. 1995:1583-1596. [40] Beer JH, Springer KT, Coller BS, et al. Immobilized Arg-Gly-Asp (RGD) peptides of varying lengths as structural probes of the platelet glycoprotein IIb/IIIa receptor. Blood. 1992;79(1):117-128. [41] Silver JH, Hergenrother RW, Lin JC, et al. Surface and blood-contacting properties of alkylsiloxane monolayers supported on silicone rubber. J Biomed Mater Res. 1995; 29(4):535-548. [42] Wang CC, Tsai H, Shih HH, et al. Synthesis and characterization of poly(ethylene glycol) derivatives. J Poly Sci Poly Chem Ed. 1984;22(2):341-352. [43] Sawhney AS, Hubbell JA. Poly (ethylene oxide)-graft-poly(L-lysine) copolymers to enhance the biocompatibility of poly(L-lysine)-alginate microcapsule membranes. Biomaterias. 1992;13(12):863-870. [44] Kobayashi H, Ikada Y. Covalent immobilization of proteins on to the surface of poly(vinyl alcohol) hydrogel. Biomaterials. 1991;12(8):747-751. [45] Bannwarth W, Schmidt D, Stallard RL, et al. Bathophenanthroline-ruthenium(II) complexes as non-radioactive labels for oligonucleotides which can be measured by time-resolved fluorescence techniques. Helvetica Chimica Acta. 1988;71(8):2085-2099. [46] Liu SQ, Ito Y, Imanishi Y. Cell growth on immobilized cell growth factor. 9. Covalent immobilization of insulin, transferrin, and collagen to enhance growth of bovine endothelial cells. J Biomed Mater Res. 1993;27(7): 909-915. [47] Streeter HB, Rees DA. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987;105(1):507-515. [48] Werb Z, Tremble PM, Behrendtsen O, et al. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;(2):109:877-889. [49] Jagendorf AT, Patchornik A, Sela M. Use of antibody bound to modified cellulose as an immunospecific adsorbent of antigens. Biochim Biophys Acta. 1963; 78(3):516-527. [50] Tseng YC, Park K. Synthesis of photoreactive poly(ethylene glycol) and its application to the prevention of surface-induced platelet activation. J Biomed Mater Res. 1992;26:373-391. [51] Yan MD, Cai SX, Wybourne MN, et al. Photochemical functionalization of polymer surfaces and the production of biomolecule-carrying micrometer-scale structures by deep-UV lithography using 4-substituted perfluorophenyl azides. J Am Chem Soc. 1993;115(2): 814-816. [52] Guire, Patrick E. Biocompatible device with covalently bonded biocompatible agent, United States Patent 5263992. 1993. [53] Yoshiaki S, Masahiro K, Masaya I. Surface analysis of antithrombogenic ion-implanted silicon rubber. Nucl Instrum Methods Phys Res B. 1991;59-60(Part 2): 1300-1303. [54] Steele JG, Johnson G, Norris WD, et al. Adhesion and growth of cultured human endothelial cells on perfluorosulphonate: pole of vitronectin and fibponectin in cell attachment. Biomaterials. 1991;12(6):531-539. [55] Klein-Soyer C, Hemmendinger S, Cazenave JP. Culture of human vascular endothelial cells on a positively charged polystyrene surface, primaria: comparison with fibronectin-coated tissue culture grade polystyrene. Biomateriala. 1989;10(2):85-90. [56] Rémy M, Bordenave L, Bareille R. Endothelial cell compatibility testing of various prosthetic surfaces. J Mater Sci. 1994;5(11):808-812. [57] Xu L, Jing ZP. Artificial vascular graft endothelialization of natural. Shanghai Shengwu Yixue Gongcheng Zazhi. 1997;18(2):31-32. [58] Jing ZP, Xu L. Experimental study of emdothelialization on domestic silk dacron graft. Shanghai Shengwu Yixue Gongcheng Zazhi. 1998;19(1):19-20. [59] Marko T, Peter B. Vascular graft wall. Eur Paitent. EP0248247, 1987. [60] Chen BL, Wang DA. Preparation and mechanism of anticoagulatent biomedical polymer materials with blood compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2011; 15(29):5507-5510. [61] Chen BL, Wang DA, Feng LX. Investigation on methods of surface modification of tissue engineering materials: polymer surface group transformation and bioactive molecule immobilization. Zhongguo Zuzhi Gongcheng Yanjiu. 2010;14(3):552-554. [62] Chen BL, Wang DA, Feng LX. Surface modification of tissue-engineered materials plasma and grafting modification. Zhongguo Zuzhi Gongcheng Yanjiu. 2009; 13(3):587-590. [63] Chen BL, Wang DA, Feng LX. Application of polymer biomaterials in the tissue engineering. Zhongguo Zuzhi Gongcheng Yanjiu. 2008;12(6):1189-1192. [64] Chen BL, Wang DA, Feng LX. Polymer porous membrane prepared using thermally induced phase separation. Zhongguo Zuzhi Gongcheng Yanjiu. 2007; 11(40):8217-8219. [65] Chen BL, Wang DA, Feng LX. Topology of tissue engineered material surface for cell compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11(18): 3653-3656. [66] Chen BL, Wang DA, Feng LX. Effects of physical-chemical properties of tissue engineered material surface on cell compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11(1):197-200. [67] Chen BL, Wang DA, Feng LX. Cytological effect of tissue engineering materials with cell compatibility. Zhongguo Linhchunag Kangfu. 2006;10(45):225-227. [68] Chen BL, Wang DA, Feng LX, et al. The application of biomedical tissue engineering and the polymer tissue engineering material. Gaoshi Like Xuekan. 2007;27(1): 24-26. [69] Chen BL, Wang DA, Feng LX, et al. Study on the blood compatibility of biomedical ploymer materials-project of antithromboeicity materials. Suihua Xueyuan Xuebao. 2007;27(1):186-188. [70] Chen BL, Wang DA, Feng LX, et al. Study on surfaces modify of the tissue engineering materials and application in the tissue engineering. Hulunbeier Xueyuan Xuebao. 2007;15(1):52-54. [71] Chen BL, Wang DA, Feng LX, et al. Study on the tissue compatibility of biomedical ploymer materials-project of tissue-compatibility materials. Hulunbeier Xueyuan Xuebao. 2006;14(6):34-36. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Jiang Xin, Qiao Liangwei, Sun Dong, Li Ming, Fang Jun, Qu Qingshan. Expression of long chain non-coding RNA PGM5-AS1 in serum of renal transplant patients and its regulation of human glomerular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 741-745. |
| [5] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [6] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [7] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [8] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [9] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [10] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||